Univ.-Prof. Dipl.-Ing. Dr.rer.nat. Ulrich Stelzl
+43 316 380 - 5397
Institut für Pharmazeutische Wissenschaften
nach Vereinbarung (ext. 5365)
ORCID: https://orcid.org/0000-0003-2500-3585
In the section of Pharmaceutical Chemistry, we are scientifically investigating and teaching our students on how drugs and new drug candidates are made, how to detect and quantify drugs and their metabolites, how drugs act on their targets and if/how we can improve their mode/range of action. The Institute is sub-structured into five working groups:
News
We are part of MOBILES
News on uni.on (German only)
Announcements of the Institute of Pharmaceutical Sciences
Research summary
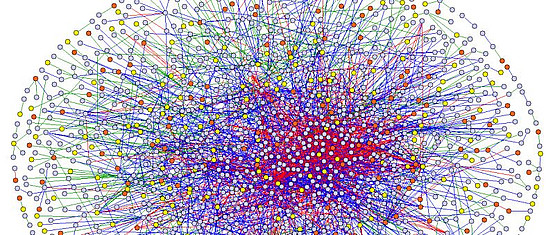
The group is focusing on the analysis of molecular interaction networks with the aim to understand the dynamics of molecular networks underlying cellular processes related to human disease. Experimental functional genomics techniques, e.g. HTP Y2H screening, are utilized in combination with biochemical, cell biological and computational methods.
Current DNA sequencing approaches and mass spectrometry based proteomics technologies allow the simultaneous measurements of gene/protein variants for series of cell types, conditions or disease states. In particular the discovery of new somatic mutations in health and disease has vastly outpaced our ability to assess their functional roles. As most of the variation alone is not causal with respect to the observed phenotype, the question arises how to systematically analyze the large number of molecular variation and how to assess the importance of combined changes for cellular function, disease development and drug action? In our work we focus on the systematic analyses of the functional impact of genetic variation and post-translational protein modification on protein-protein interaction, which as universal protein function underlies cellular phenotypes. Using deep scanning mutagenesis approaches we bridge the knowledge gap between nucleotide resolution genomics and protein resolution proteomics.
Network biology offers a more comprehensive understanding of biological processes. Advances in systems biology indicate that complex diseases may not be treated effectively by interventions at single drug targets and with single drugs. Network biology will advance our understanding of drug action and will be an important piece in developing individualized medicine.
Recommended reading on interaction networks / network biology / network pharmacology:
Systematic interaction studies in human disease research, a technology feature free at Nature magazine: Protein maps chart the causes of disease by Marissa Fessenden (Bozeman|Montana)
A new discipline: network science !? An insight commentary at Nature Physics suggests ... The network takeover by Albert-László Barabási (Boston|Massachusetts)
Publications
Updated research records can be found at
PMC Europe, NCBI biography, pubmed, google scholar, scopus or WoS
Recent selected publications
Kohlmayr JM, Grabner GF, Nusser A, Höll A, Manojlović V, Halwachs B, Masser S, Jany-Luig E, Engelke E, Zimmermann R, Stelzl U (2024);
Mutational scanning pinpoints distinct binding sites of key ATGL regulators in lipolysis;
Nat Commun 15, 2516; doi: 10.1038/s41467-024-46937-x [OA]
Moesslacher CS, Auernig E, Woodsmith J, Feichtner A, Jany-Luig E, Jehle S, Worseck JM, Heine CL, Stefan E, Stelzl U (2023);
Missense variant interaction scanning reveals a critical role of the FERM domain for tumor suppressor protein NF2 conformation and function;
Life Sci Alliance 6: 202302043; doi: 10.26508/lsa.202302043 [OA]
Smolnig M, Fasching S, Stelzl U (2023);
De Novo Linear Phosphorylation Site Motifs for BCR-ABL Kinase Revealed by Phospho-Proteomics in Yeast;
J Proteome Res 22: 1790–1799; doi: 10.1021/acs.jproteome.2c00795 [OA]
Jehle S, Kunowska N, Benlasfer N, Woodsmith J, Weber G, Wahl MC, Stelzl U (2022);
A human kinase yeast array for the identification of kinases modulating phosphorylation-dependent protein-protein interactions;
Mol Syst Biol 18: e10820 ; doi: 10.15252/msb.202110820
article link at EMBO press
Kunowska N, Stelzl U (2022);
Decoding the cellular effects of genetic variation through interaction proteomics;
Curr Opin Chem Biol 66: 102100 ; doi: 10.1016/j.cbpa.2021.102100
article link at Sciencedirect
Link to the website of the working group
Ass.-Prof. Dipl.-Ing. Dr.techn. Jürgen Hartler
ao. Prof. Dr. Mag. Andreas J. Kungl
Research Summary
In our laboratory we mainly focus on the structural and functional principles of protein-ligand interactions, which are used for the discovery and development of novel drugs. We focus our work on therapeutically relevant ligands, so-called glycosaminoglycans (GAGs), which represent a group of linear, highly charged polysaccharides involved in many (patho)physiological processes such as inflammation, cancer, infection, etc. GAGs are part of the extracellular matrix, which acts as an interface for interactions of tissues with other cells such as immune cells, tumor cells, and microorganisms. These interactions are usually mediated by cytokines and growth factors. These are small signaling proteins that serve as a template for our protein engineering protocols, which are designed to develop novel biopharmaceuticals for the treatment of chronic inflammatory diseases - such as COPD, esophagitis, lupus nephritis and cystic fibrosis - as well as cancer and viral infections such as SARS-CoV-2. To achieve our long-term goals, we collaborate with several other academic institutions and pharmaceutical/biotech companies in various fields (see the list of our collaborators). In addition, we try to turn our research into new therapies ourselves through our own spin-out companies (such as ProtAffin Biotechnologie AG and Antagonis Biotherapeutics GmbH).
Antagonis is a privately held biotech company headquartered in Graz, Austria, developing protein-based glycosaminoglycan antagonists. Glycosaminoglycans (GAGs) are involved in a variety of diseases such as inflammation, fibrinogenesis, wound healing, immuno-oncology & metastasis, blood clotting, myocardial infarction & restenosis, multiple sclerosis & neurodegeneration. These highly charged and linear polysaccharides represent a novel group of therapeutic targets that cannot serve as antigens for the development of conventional biologics such as monoclonal antibodies due to their problematic preparation. Antagonis is therefore using the recognition sites of naturally GAG-binding proteins - such as those of chemokines - and developing them towards increased GAG-binding affinity while maintaining selective ligand recognition. For candidate selection/optimization, a unique collection of human GAG oligosaccharides was generated, which will be used to screen and optimize our GAGbodies. Target accuracy and potential target miss effects are tested using the proprietary ELICO technology. In addition, an extensive set of GAG-binding proteins has been collected in our glyaffibase, which serves as the basis for our targeted GAGbody engineering. A patent has recently been granted for the GAGbody technology in Europe and the United States.
Biopharmaceuticals / Biologics
Drug Development
Ao.Univ.-Prof. Mag.pharm. Dr.rer.nat. Robert Weis
Natural product synthesis
Ass.-Prof. Mag.pharm. Dr.rer.nat. Armin Presser
Drug development and gender pharmacy
Associate Professor Mag.pharm. Dr.rer.nat. Edith Gößnitzer
Drug Synthesis
Ass.-Prof. Dipl.-Chem. Dr.rer.nat. Antje Hüfner
Drug and Drug Analysis Bio Sensors NMR Center
Development of methods for enantiomer separation by High Performance Liquid Chromatography (HPLC) and Capillary Electrophoresis (CE). This topic involves synthesis of new chiral selectors for HPLC , CE and the new principle of capillary electrochromatography (CEC); Synthesis of chiral phases, development of new chiral separation principles; application to the separation of the enantiomers of compounds of biological interest, drugs and pestizides. Development of Flow-Injection Immunoassays (FIIA). Flow-Injection Immunoassays represent a new, rapid immunoassay technique making use of Flow-Injection Analysis systems. Hetero-and homogeneous assays using fluorescence- and chemiluminescence detection are beeing developed. Application of these systems for pharmaceutical analysis, environmental analysis and diagnostic purposes are under investigation. The main interest of our research work is focussed on the development of methods for the determination of biological active compounds in the picomole or femtomole range in biological materials. This includes pharmacokinetic studies of drugs and metabolites in blood, plasma, serum and urine as well as pesticide quantification in water and other materials, analysis of cosmetic products, or prostaglandin and phospholipid determination in different human materials. In order to enhance sensitivity and selectivity derivatization agents for flourescence or electrochemical detetion in combination with chromatographic separation techniques are beeing designed. Photolytic processes and recently, problems dealing with clinical pharmacy are under investigation. Further projects are centered around modern analytics of pumpkin seeds and their oil as well as sterol analysis of eatable oils.
Research focus Pharmaceutical Analysis: Design of electrochemical (bio)sensors for determining biologically active substances in complex matrix and as screening tools for diagnostic applications. The sensors are based on carbon paste and screen printed electrodes. Development of HPLC and FIA methods for the quantification of drugs and their metabolites and/or degradation products within the context of stability tests, quality control and pharmakokinetic studies. Detection is mainly carried out by fluorimetric and electrochemical techniques. Example: Principal of developed PSAO-biosensors for analyzing biogenic amines in food. Cooperations Prof. Dr. Dale Edmondson Prof. Dr. Kurt Kalcher Prof. Dr. Peter Macheroux Prof. Dr. Anton Sadjak Prof. Dr. Manfred Schubert-Zsilavecz Dr. Mario Wurglics
Clinical Pharmacy Medicinal product related investigations for improving patient safety in the clinical area. Example: Investigation of the susceptibility of errors in dispensing and administering of drugs.
Dr. Ian Rudd Dr. Lam Sin Hoon Dr. Ingrid Friedel Dr. Marianne Leitner |
Biological aspects of natural and synthetic products against cellular stress and tissue disorders
Univ.-Prof. Dr.rer.nat. Valery Bochkov
Ass.-Prof. Dipl.-Ing. Dr.techn. Olga Oskolkova
Mag.pharm. Dr.rer.nat. Bernd Gesslbauer
Priv.-Doz. Dr.rer.nat. Hanna Engelke
Main research interests:
- Development of cellular models for screening of natural products as well as synthetic substances that have anti-inflammatory and cell-protective effects.
- Investigation of cellular protective mechanisms triggered by stress-inducing substances
- Search for new targets to prevent cellular as well as tissue damage induced by oxidized phospholipids.
Cooperation partner:
- Prof. Dr. Therese Resink, University of Basel
- Prof. Dr. Konstantin Birukov, University of Chicago
Link to the website by Assoc. Prof. Dr.rer.nat. Hanna Engelke