Group Leader

Univ.-Prof. Dr.rer.nat. Sebastian Preissl
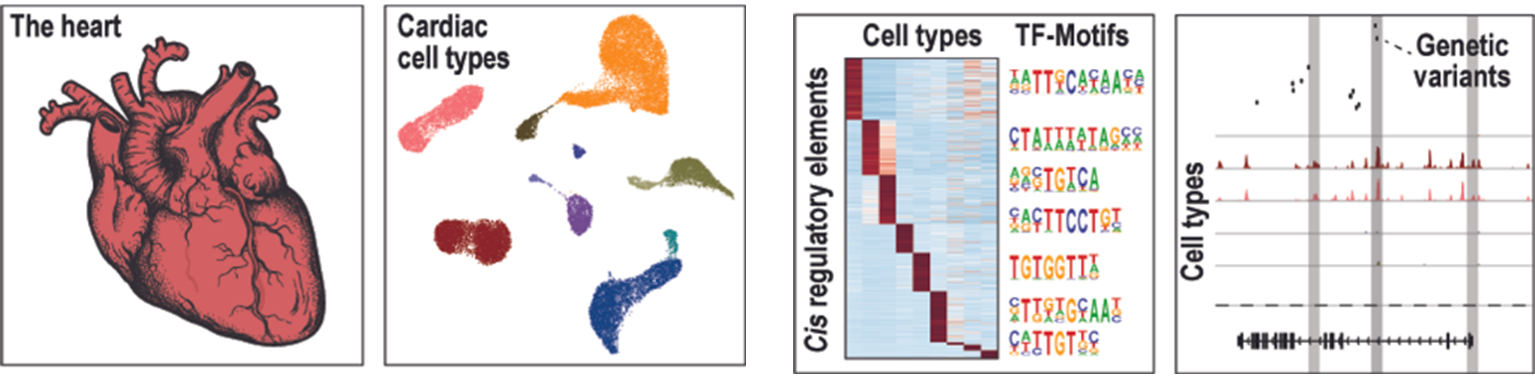
Cardiovascular Diseases
Cardiovascular diseases (CVDs) including heart failure and atrial fibrillation are major leading causes of morbidity and mortality worldwide. Our research aims to identify and characterize transcriptional and epigenetic factors, molecular pathways as well as regulatory elements, e.g. enhancers as potential therapeutic targets for cardiovascular diseases.
We use a combination of single cell epigenomic/multiomics technologies, mouse models and functional perturbation in cell culture models to (1) untangle and modify cardiac cell-type resolved gene regulation, (2) to dissect cell-cell communications between cardiac cell types, and (3) to reveal the molecular function of genetic variants associated with cardiovascular diseases.
We hope that our research can help to develop new personalized therapeutic strategies for CVDs.
Part of this project is a subproject of DFG-funded GRK 2344: MeInBio – BioInMe and supported by the DFG-funded CRC1425 Heterocellular Nature of Cardiac Lesions: Identities, Interactions, Implications.
Autosomal dominant polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic cause of kidney failure, affecting over 12 million people. Mutations in PKD1 and PKD2 lead to cyst formation in individual renal epithelial cells, resulting in cellular remodeling and immune cell infiltration. The aim of this project is to identify gene regulatory programs underlying these remodeling processes. We investigate disease initiation and progression at defined time points in a mouse model and analyze cell type-specific gene regulatory programs as well as cellular interactions during cyst development and disease progression.
This project is a subproject of DFG-funded CRC1453: Nephrogenetics (NephGen) jointly with Prof. Dr. Michael Köttgen (Universität Freiburg, Deutschland).
Perinatal Development of Macrophages
Tissue macrophages (Mφ) are the most abundant skin resident immune cell type. Their diverse tasks range from resistance against pathogens to tissue repair. The project focuses on factors that influence dermal macrophage (Mφ) diversity during the perinatal development. Dermal Mφ differ in ontogeny, gene expression profiles, and localization. Adaptation of Mφ depends on a complex interplay of molecular factors including epigenetic processes. In this project, we aim to obtain a comprehensive understanding (1) of the dermal Mφ subtypes and their tissue niches, 2) of the gene regulatory programs underlying Mφ adaptation, and 3) to investigate their influence on the establishment of dermal immunity.
This project is a subproject of DFG-funded TRR359: Perinatal Development of Immune Cell Topology (PILOT) jointly with Dr. Julia Kolter (Universität Freiburg, Deutschland).